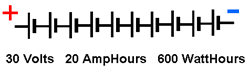Battery and Energy Technologies |
 |
|
Battery Beginners PageThis page provides some basic information to help students with their physics homework as well as to satisfy the curiosity of casual non-technical visitors who have stumbled upon this site by mistake.. Links are provided to pages where more detailed information can be found.
CellsEnergy cells are the smallest individual electrochemical unit which delivers a voltage which depends on the cell chemistry. Examples are cylindrical alkaline cells used in toys and small electronic devices. They may be primary (single use) cells or they may be secondary (rechargeable) cells. Strictly speaking, a cell should not be called a battery since a battery is a group of cells but many people (including me at times) use the word "battery" to describe any electrochemical energy source, even if it is a single cell, and this can lead to confusion. Energy cells provide a DC or Direct Current (unidirectional) source of electricity.
BatteriesBatteries and Battery Packs are made up from groups of cells, sometimes designed into a single block as in 12 Volt automotive batteries which are made up from six 2 Volt cells connected in series and integrated into a single unit. Or they may be individual cells wired together in a separate case.
Cell VoltageThe cell voltage depends on the combination of active chemicals used in the cell. For commonly available cells the voltage can range from 1.2 Volts for Nickel based cells to over 3 Volts for Lithium based cells.
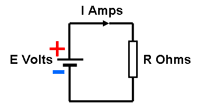
Battery CurrentThe actual current delivered by the cell or battery at any particular instant depends on the load.
Ignoring the effect of the battery's internal resistance, the current drawn by the load is given by I = E ÷ R Where I is the current (Amps), E is the battery or cell voltage (Volts) and R is the load resistance (Ohms). This relationship is known as Ohm's Law Thus for a 2 Volt battery supplying a 2 Ohm load, the current will be 1 Amp.
The C rate is a measure of the battery's current handling capability. It is NOT the maximum current which the battery can deliver which may be specified by the manufacturer as several times the `C rate. It is the constant current charge or discharge rate which the battery can sustain for one hour. Thus a 12 Volt, 20 AmpHour battery should be able to deliver 20 Amps for 1 hour or 2 Amps for 10 hours. If the battery is dischaged at the 10 C rate, it will be completly discharged in 6 minutes. The charging C rate is normally less than the discharge C rate.
Internal ImpedanceThe chemicals and current carrying conductors in practical batteries have a small internal resistance which impedes the current flow through the battery. In the diagram below this is shown as resistance r between the battery terminals.
With no load resistance R on the battery, the open circuit voltage at the battery terminals will be E Volts. In this case however, when a load resistance R is connected across the battery, the current flowing will be I = E ÷ (R + r) and there will be a voltage drop across the internal resistance. Also known as the Ohmic loss, the voltage drop e is given by e = I r = E r ÷ (R + r) The consequence is that the available voltage at the battery terminals is reduced to (E - e) = E - ( E r ÷ (R + r)) or E (1- r / (R+r)) Thus for a 2 Volt battery with an internal resistance of 100 milliOhms feeding a load of 2 Ohms, the operating voltage at the battery terminals will be only 1.9 Volts and the current through the load will be 0.95 Amps.
It appears as if a small voltage e is being applied inside the battery in the opposite direction to the battery voltage. Note that e is dependent on the magnitude of the current flowing. For most batteries the internal resistance r is very small, only a few milliOhms, so its effect can be neglected, but for high power batteries the effect of internal resistance can be quite significant causing the battery to heat up due to Joule heating (see below) as well as an equivalent reduction in available power. See more about the effects of internal impedance. Charge, Energy and PowerCharge: The unit of Electric Charge is the Coulomb. One Coulomb is equal to the charge transferred by a current of one ampere in one second. Energy and Work Done: Energy is the capacity to do Work. Energy and Work Done are both measued in Joules or WattHours.1 Joule = 1 WattSecond. See also Glossary (Joule) Energy purchased from the electricity utility, (in this case Alternating Current or AC) is commonly measured in "Units" where 1 Unit = 1 KiloWattHour or 1000 WattHours. Power: Power is the Rate of doing Work. It is measured in Watts. 1 Watt = 1 Joule per Second.
Battery PowerThe power which a cell or battery can deliver is normally specified as the power associated with drawing current at the C rate. The actual power delivered however depends on the load resistance as above and is given by: P = E X I Where P is the power delivered (Watts) Thus for a 20 AmpHour 12 Volt battery the power provided is given by; 20 Amps X 12 Volts = 240 Watts The power dissipated in the load appears as heat and is given by: P= I2R This equation also represents the process known as Joule Heating
Battery Capacity and Energy Content (They are not the same)
As noted above, the cell or battery current handling capacity is normally specified in AmpHours or MilliampHours and represents the current in Amps or Milliamps which can be sustained by the battery for one hour. This is known as the "C" Rate of the battery, but this measure of charge capacity is confusingly used as an indication of the battery's energy storage capacity, without taking account of the cell or battery voltage.
The rate at which charge is transferred into or out of a cell or battery is simply the current I. The amount of charge transferred by the current is measured in Coulombs and is given by Q = I X t Where Q is the quantity of charge transferred and t is the time in seconds that the current flows. The quantity of charge in a fully charged cell, its Coulomb capacity, is therefore given by the AmpHour capacity multiplied by 3600, (the number of seconds in an hour) no matter what the battery voltage is. Thus a fully charged 20 AmpHour capacity battery contains, or can deliver a charge of: 20 AmpHours X 3600 Seconds = 72,000 Coulombs AmpHours and Coulombs are thus equivalent measures of a battery's charge capacity.
The actual current which flows into the load depends on the battery voltage and this is different for different cell chemistries as shown in the following table. Note that although all the batteries may contain the same amount of charge, when connected to a similar load (2 Ohms in this example) the higher the cell voltage, the more current which flows and the quicker the battery is discharged.
The standard C Rate refers to a specified constant current flowing in the battery during a reference charge or discharge period of one hour. Typical current flow rates may be much higher or lower than the standard C Rate, resulting in correspondingly lower or higher charge or discharge periods for the same battery. For convenience, multiples of the C Rate may be used to represent the actual application. They do not change the capacity of the battery (unless they drive it beyond its specified limits).
By convention the C Rate notation representing the alternative current drain shows the current multiple appended to the C Rate. Thus a C3 current drain will discharge the battery at 3 times the rate in one third of an hour and a charging rate of C0.5 will take 2 hours to charge the battery at half the C Rate. The table opposite shows the discharge currents and periods for a 20 AmpHour 12 Volt battery with a specified C Rate of 20 Amps when operated at different C Rates.
The AmpHour or Coulomb capacity is not a measure of the energy content of the battery. The energy stored in a cell or battery also depends on the voltage and is specified in WattHours or MilliwattHours. To get the cell or battery energy content, multiply the AmpHour rate by the cell or battery voltage to obtain the WattHours. In the above example the energy in a 12 Volt, 20 Ah battery is given by: 20 AmpHours X 12 Volts = 240 WattHours
When choosing batteries for battery powered applications, the key requirements are the amount of energy which needs to be stored to supply the application and the voltage and current at which it is delivered. The energy content however depends on the battery voltage and this is different for different cell chemistries. Comparing batteries by their AmpHour capacity can lead to misleading conclusions since they may all have the same AmpHour capacity but the energy content may be different as shown in the table below. For completeness the energy content of the battery is given in Joules as well as Watthours. (1 Joule = 1 WattSecond or 1 WattHour = 3600 Joules)
For an appreciation of the amount of energy stored in a battery see What a Joule can do.
Beware that the actual energy obtained from the battery may not match exactly the specified capacity even when operating at the specified C Rate. This is because the battery voltage tends to fall towards the end of its discharge cycle resulting in lower discharged energy. See Discharge Curves
Beware also that the performance of most batteries is temperature sensitive, so that the energy which can be extracted from the battery tends to be reduced as the temperature falls. See Temperature Performance. The battery will only provide its specified energy when operated at its specified temperature.
The answer is that in the higher voltage battery, the charge is stored at a higher potential. Compare the example with two identical canisters of water, both containing the same quantity of water, but one contains water at atmospheric pressure and the other contains water under high pressure.
For a given size, primary cells normally have a higher energy content (capacity) than secondary cells but they are exhausted and no longer usable after a single discharge. Though secondary cells generally have a lower capacity per charge they can be discharged and recharged many times over, thus increasing their effective energy throughput during their lifetime to many times the capacity of primary cells of equivalent size.
How Batteries WorkSee details on the Cell Chemistries page.
Battery PricesPricing batteries by the AmpHour inevitably leads to confusion, particularly when batteries with different cell chemistries are being compared, because the energy content depends on the battery voltage. The actual stored energy in WattHours is equal to the AmpHour capacity multiplied by the battery Voltage.
To compare the cost of equivalent amounts of stored energy on a like for like basis, the price per WattHour should be used. To convert the price per AmpHour to the price per WattHour, divide the AmpHour price by the battery Voltage.
Example A 12 Volt 60 AmpHour Lead acid battery selling for $90.00 costs $1.50 per AmpHour or $0.12 per WattHour A 3.7 Volt (18650) consumer Lithium cell as used in laptop computers delivers 2.25 AmpHours and sells for about $4.00 which is equivalent to $1.77 per Amphour or $0.48 per WattHour
Thus the Lithium battery price per AmpHour is only slightly more than the AmpHour price of the Lead acid battery but the cost of the energy supplied by the Lithium battery is four times the cost of the same amount of energy delivered by the Lead acid battery. But this is not the whole story. This example only considers the capital cost of the battery. True cost comparisons should aslo take into account the battery cycle life.
Cycle LifeThis is the number of times a rechargeable battery can be charged and discharged before it wears out. It depends on the cell chemistry and the operating conditions under which the battery is used.
A Lead acid battery may be expected to have a cycle life of around 300 cycles, whereas a Lithium battery with the same capacity may be capable of 1,500 cycles before needing replacement. A long term application requiring a given charge capacity per cycle could be satisfied by a single Lithium battery, or a series of five Lead acid batteries (the initial battery plus four repacements). We showed in the example above that Lithium batteries could be four times more costly per WattHour than Lead acid batteries, but since they have a superior cycle life (5 times better), the Lead acid alternative turns out to be 25% more expensive.
Cycle life is a particularly important consideration when comparing the lifetime costs of expensive batteries such as those used in electric vehicle applications.
Series ConnectionsWhen a battery is constructed from group of cells connected in series, the battery voltage is the sum of the voltages of the individual cells, but the AmpHour capacity is the same for the chain since the same current passes through all of the cells.
Thus a battery constructed from 10 X 3 Volt X 20 AmpHour cells will have a battery voltage of 30 Volts and an AmpHour capacity of 20 AmpHours. It will have a true capacity of 600 WattHours of energy and will be able to deliver 600 Watts of power.
Note that a single 3.6 Volt 800 mAh Lithium cell stores he same energy (2.88 Wh) as three 1.2 Volt 800mAh NiCads or Nickel Metal Hydride cells.
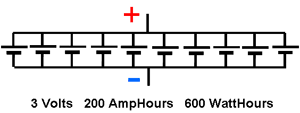
Parallel ConnectionsWhen the same 10 cells are connected in parallel, the battery voltage is the same as the voltage of the single cells, but the Amphour capacity will be the sum of the AmpHour capacity of the cells since the current through the load is the sum of all the currents through the individual cells.
Thus the battery will have a voltage of 3 Volts and an AmpHour capacity of 200 AmpHours. It will still have a true capacity of 600 WattHours of energy and will be able to deliver 600 Watts of power as in the case above.
Looking at this another way; each cell has an energy storage capacity of 60 WattHours. The 10 cells will have a combined capacity of 600 WattHours no matter how they are connected. Similarly the available power will always be 600 Watts. The power is given by the current multiplied by the voltage. A series configuration thus provides a high voltage but low current and a parallel configuration gives a high current but at low voltage.
Batteries for any current and voltage rating can be made up from combinations of series and parallel connections of small cells.
BUT. Batteries should not be made up from mixed cells. Do not mix cells of different ages, different sizes, different voltages, different chemistries, different capacities, different shapes or different manufacturers. Mixing cell types within a battery can lead to some cells being overloaded leading to early failure and this could be dangerous.
See also Battery Safety Instructions
Battery ChargingThe methods used for battery charging are specific to the type of cell chemistry used in the battery. This means that apart from using the appropriate voltage and current ratings, the charging profile must be tailored to the cell chemistry, otherwise the battery may be damaged. See more on the page about Chargers and Charging.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
![]() Print This Page || Home || FAQ || Site Map || Legal || Privacy Promise || Contacts
Print This Page || Home || FAQ || Site Map || Legal || Privacy Promise || Contacts
Woodbank Communications Ltd, South Crescent Road, Chester, CH4 7AU, (United Kingdom)
Copyright © Woodbank Communications Ltd 2005